Dispersion forces, also known as London forces, are the temporary attractive force that results when electrons in two adjacent atoms take positions that make the atoms form temporary dipoles. The dispersion forces cause non polar substances to condense to liquids and freeze to solids when the temperature is lowered. When two non polar molecules are close to each other, the electron cloud of one molecule repels the electron cloud of the other molecule making the density around each nucleus greater in one region of each cloud. This makes each molecule form a temporary dipole. Dipole forces are known to be the weakest intermolecular forces. Here is an image on dispersion forces:
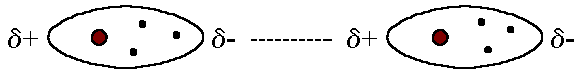 |
| Dispersion forces are present between any two molecules when they are almost touching. |
Dipole-dipole forces:
They are the forces which are attractive forces between the positive end of a polar molecule and the negative end of another polar molecule. This takes place since some regions of a polar molecule are always partially negative and other regions partially positive. Dipole-dipole forces are much weaker than ionic or covalent bonds and have and are only significant when the molecules involved are close to each other. Here is an image on dipole-dipole forces:
 |
| This image shows polar iodine monochloride (ICl) molecules that give rise to dipole-dipole attractions. |
Hydrogen bonds:
 |
| This shows the hydrogen bond that is taking place. |

We also provide analytical services and laboratory services to our customers. 1-decyl-3-methylimidazolium perfluorobutanesulfonate
ReplyDelete