Saturday, January 22, 2011
Introduction to Intermolecular Forces
Intermolecular Forces are a part of the basics of current chemistry. These forces can be summarized by the attractions and repulsions felt between atoms and molecules and differ from chemical bonds. Many people get confused with intermolecular and intramolecular forces, while internare much weaker than intra. When it comes to breaking bonds, the chemical bonds form a new substance that reacts differently due to it's different properties, while when breaking intermolecular forces, it does not change it's chemical properties or chemical reactivity. On the other hand, intermolecular forces can determine how quickly a reaction can occur or if it will take place. Intermolecular forces also determine wether a material will be a liquid, solid, or a gas at a specific temperature. There are three main types which are: Dispersion Forces, Dipole-Diopole Forces, and Hydrogen Bonds which will be explained below.
Types of intermolecular forces:
Dispersion forces:
Dispersion forces, also known as London forces, are the temporary attractive force that results when electrons in two adjacent atoms take positions that make the atoms form temporary dipoles. The dispersion forces cause non polar substances to condense to liquids and freeze to solids when the temperature is lowered. When two non polar molecules are close to each other, the electron cloud of one molecule repels the electron cloud of the other molecule making the density around each nucleus greater in one region of each cloud. This makes each molecule form a temporary dipole. Dipole forces are known to be the weakest intermolecular forces. Here is an image on dispersion forces:
Dipole-dipole forces:
They are the forces which are attractive forces between the positive end of a polar molecule and the negative end of another polar molecule. This takes place since some regions of a polar molecule are always partially negative and other regions partially positive. Dipole-dipole forces are much weaker than ionic or covalent bonds and have and are only significant when the molecules involved are close to each other. Here is an image on dipole-dipole forces:
Hydrogen bonds:
This bond is a special type of dipole-dipole attraction which occurs between molecules containing a hydrogen atom bonded to a highly electronegative atom with at least one electron pair. For this hydrogen bond to take place,a hydrogen atom must be bonded to either fluorine, oxygen, or a nitrogen atom since they are electronegative enough to cause a weak yet partially negative charge on one region of the molecule, and a partially positive charge on the other region of the molecule.
Dispersion forces, also known as London forces, are the temporary attractive force that results when electrons in two adjacent atoms take positions that make the atoms form temporary dipoles. The dispersion forces cause non polar substances to condense to liquids and freeze to solids when the temperature is lowered. When two non polar molecules are close to each other, the electron cloud of one molecule repels the electron cloud of the other molecule making the density around each nucleus greater in one region of each cloud. This makes each molecule form a temporary dipole. Dipole forces are known to be the weakest intermolecular forces. Here is an image on dispersion forces:
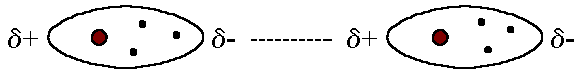 |
| Dispersion forces are present between any two molecules when they are almost touching. |
Dipole-dipole forces:
They are the forces which are attractive forces between the positive end of a polar molecule and the negative end of another polar molecule. This takes place since some regions of a polar molecule are always partially negative and other regions partially positive. Dipole-dipole forces are much weaker than ionic or covalent bonds and have and are only significant when the molecules involved are close to each other. Here is an image on dipole-dipole forces:
 |
| This image shows polar iodine monochloride (ICl) molecules that give rise to dipole-dipole attractions. |
Hydrogen bonds:
 |
| This shows the hydrogen bond that is taking place. |
You ask...We explain
In this section, some students have sent us questions on intermolecular forces, and we have joyfully answered them. Leave a comment if you have any questions or would like to say something.
Questions
Question 1: Nitrgen compromises 78% of the atmosphere while oxygen compromises 21%. These two gases have low boiling points and therefore can exist as gases under normal environmental conditions. How can the intermolecular forces that exist between the nitrogen molecules or the oxygen molecules explain the low boiling points? (Asked by Paolo from Spain)
Question 2: The hydrogen sulfide molecule (H2S) and the water molecule (H2O) are very similar. However, the boiling point of hydrogen sulfide is -60 degrees Celsius whereas the boiling point of water is 100 degrees Celsius. How can the intermolecular forces explain this difference? (Asked by Biggie from West Coast)
Question 3: Iodine solid sublimes to iodine gas. What happens in terms of intermolecular forces as this process occurs? (Asked by Chantal from Mansourieh)
----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Answers
Answer 1: In molecules, the stronger the intermolecular forces, the higher the boiling points. In this case, the intermolecular forces must be weak, or in other words the atoms are far apart. This will result in a low boiling point since not a lot of energy is required to break the bonds. That is why nitrogen and oxygen can exist as gases under normal environmental conditions.
Answer 2: As previously said, the stronger the intermolecular forces, the higher the boiling point. So as a result, the weaker the intermolecular forces, the lower the boiling point. H2O and H2S are similar molecules. They have the same molecular geometry and are both polar. But since the oxygen atom in water is much more electronegative than the sulfur atom in hydrogen sulfide, it will result in a much more polar bond. In other words, the H2O molecule has much stronger bonds than the H2S molecule. Since the bonds of H2O are stronger, the intermolecular force is also greater, so the boiling point of the molecule is high. And since the bonds of H2S are weak, the intermolecular force is also weak, resulting in the boiling point to be much lower.
Answer 3: Sublimation occurs when a molecule changes from its solid state to its gaseous state without going through the liquid state. As the iodine solid sublimes to gas, its intermolecular forces weaken throughout the process. When it first starts, its intermolecular forces are strong, and as it reaches to its gas state, the intermolecular forces diminish, resulting with no intermolecular forces.
Thank You!
Liam Laba, Dimitri Majdalani, Hadi Haikal, Roy Karam, George Ghosn, and Sadecc Choucaire would like to thank you for browsing through our blog. We hope you enjoyed it :)
Subscribe to:
Comments (Atom)



